Deuterium and Why Grass-fed Beef is superior to Grain-finished Beef
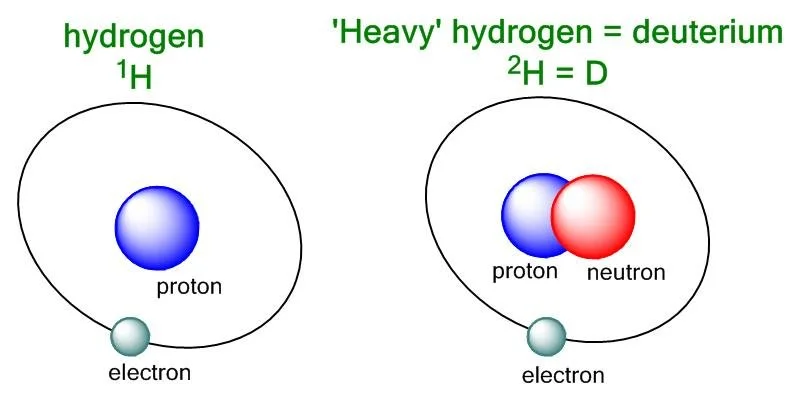
Water, which is also known as H2O, is made up of two hydrogen atoms (H) and one oxygen atom (O). The hydrogen atoms in water are mainly composed of protium, which is the lightest and most abundant isotope of hydrogen. Protium contains only one proton in its nucleus.
Deuterium, on the other hand, is an isotope of hydrogen that is heavier than protium because it contains one proton and one neutron in its nucleus. It is symbolized as 2H or D. When deuterium replaces protium in a water molecule, it creates what is called heavy water or deuterium oxide (D2O).
There is a significant difference between normal water and deuterium, and it lies in the atomic weight of the hydrogen atoms. Protium has an atomic weight of approximately 1 atomic mass unit (amu), while deuterium has an atomic weight of about 2 amu, which means that it is twice as heavy as protium.
When deuterium replaces protium in biochemical reactions, it can have a noticeable impact due to its increased atomic weight. The heavier deuterium atom can affect the vibrational motion of chemical bonds, which can alter the reaction rates. Enzymes, which are biological catalysts that facilitate biochemical reactions, can be sensitive to changes in the mass of the atoms involved.
Deuterium has a larger relative change in atomic weight compared to other isotopes like protium. This leads to a phenomenon known as the kinetic isotope effect that influences reaction rates. Typically, heavier isotopes tend to cause slower reaction rates. These effects can impact various biological processes, including metabolic pathways and cellular functions.
The kinetic isotope effect is more noticeable when using deuterium as it has a much greater mass compared to other isotopes typically present in biological molecules. This effect is especially important in research that involves deuterium labeling, as it helps trace the pathways of molecules in biological systems.
Cattle Feed and Deuterium-Rich Diets
Consuming low-quality cattle feeds may lead to the production of intracellular water that is rich in deuterium, which can pose health risks for both the animals and humans who consume the resulting products. As I mentioned, its elevated presence in metabolic processes has been associated with various health concerns.
Cows are often given low-quality feeds like grains that lack essential nutrients and may contain harmful contaminants. These feeds can cause imbalances in the cows' health and metabolic processes, especially those related to the breakdown of glycogenic substances. As a result, deuterium-enriched metabolic water is produced within the cows' cells.
During metabolic processes, glycogenic substrates such as sugars and carbohydrates are broken down to provide energy for animals. As a result of this breakdown, deuterium is incorporated into the metabolic water produced by cells. However, the accumulation of deuterium in the intracellular water can have detrimental effects on the health of cattle. It may impair cellular functions, reduce metabolic efficiency, and contribute to the development of health issues in animals.
In addition, the water produced in cattle, which is enriched with deuterium, becomes a part of the dairy and meat products that we consume. This means that we are indirectly introducing deuterium-rich substances into our bodies when we consume these products. High levels of deuterium in the human body have been linked to various health problems, such as disruptions in metabolic processes, increased oxidative stress, and a possibility of developing diseases like diabetes, obesity, and cancer.
Moreover, the application of glyphosate herbicide in genetically modified crops, which are frequently included in cattle diets, might worsen the problem of deuterium enrichment. Glyphosate exposure could potentially reduce the effectiveness of deuterium-depleted metabolic water production in mitochondria, the cell's energy-generating organelles.
Conclusion
To put it simply, low-quality cattle feeds can lead to the creation of deuterium-rich intracellular water inside animals, which can negatively impact their overall health. When humans consume products that come from these animals, they may also end up ingesting deuterium-enriched substances, which could increase the risk of developing various health conditions. This underscores the importance of assessing and enhancing the quality of cattle feeds to ensure the well-being of both livestock and consumers.
How to Get Help
The Center for Deuterium Depletion is an organization that is dedicated to advancing research and increasing awareness about the role of deuterium in health and disease. Led by Dr. Gábor Somlyai, the center's primary focus is on exploring the therapeutic potential of deuterium depletion in various medical conditions. This process involves reducing the levels of deuterium. The center collaborates with medical professionals, scientists, and researchers to conduct studies, provide education, and offer resources related to deuterium and its impact on metabolic health. If you want to know more, you can visit their website Center for Deuterium Depletion.
Sources:
Lech, J.C., Dorfsman, S.I., Répás, Z. et al. What to feed or what not to feed-that is still the question. Metabolomics 17, 102 (2021). https://doi.org/10.1007/s11306-021-01855-7
Boros LG, Somlyai I, Kovács BZ, Puskás LG, Nagy LI, Dux L, Farkas G, Somlyai G. Deuterium Depletion Inhibits Cell Proliferation, RNA and Nuclear Membrane Turnover to Enhance Survival in Pancreatic Cancer. Cancer Control. 2021 Jan-Dec;28:1073274821999655. doi: 10.1177/1073274821999655. PMID: 33760674; PMCID: PMC8204545.
Yaglova NV, Timokhina EP, Obernikhin SS, Yaglov VV. Emerging Role of Deuterium/Protium Disbalance in Cell Cycle and Apoptosis. Int J Mol Sci. 2023 Feb 4;24(4):3107. doi: 10.3390/ijms24043107. PMID: 36834518; PMCID: PMC9963022.